Nitrocellulose
Nitrocellulose, produced by nitrating cellulose with a mixture of nitric acid and sulfuric acid, was initially developed as gun-cotton, serving as a safer propellant in firearms than traditional gunpowder. This compound found applications as a low-order explosive in mining and photography, where it played a crucial role in early photographic emulsions in the 1860s. Nitrocellulose is soluble in organic solvents, making it suitable for lacquers, coatings, explosives, and celluloid production. Lacquers and coatings produced from nitrocellulose dissolve easily, leaving a transparent film, and have been used in furniture and musical instrument finishing. Explosive applications vary, with nitrocellulose being used in propellants and experiencing diverse applications, including in space missions. Nitrocellulose is a key component in nail polish due to its quick-drying and non-damaging properties. Additionally, it finds use in topical skin applications, laboratory procedures, diagnostic tests, and unconventional applications such as peeling coal balls and coating playing cards.
For any explosive material, there are oxidizing and reducing parts. In traditional gun powder, it's a mixture of a nitrate and charcoal particles. Reaction between those particles is slow because of the diffusion barrier. In nitrocellulose, oxidizing groups NO2 are much closer to reducing groups, carbon atoms. Reaction rate of the explosive decomposition has a smaller barrier and it is faster.
Back to Schedule 2023
Back to Schedule 2024
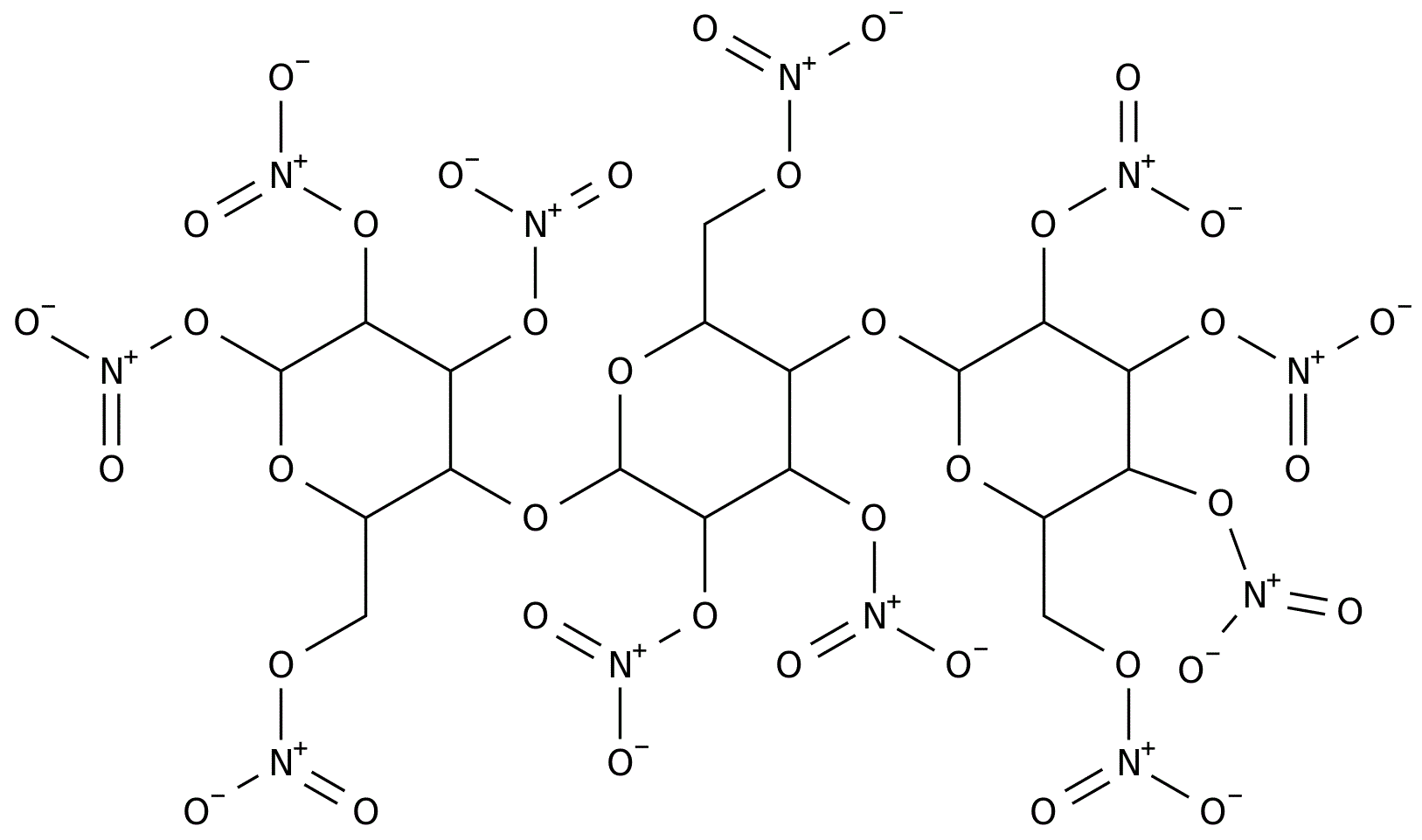
Nitrocellulose structure. Cellulose OH groups are replaced by nitro groups.